Overview
What if medicines could sense and respond to health conditions directly in your gut?
Together with Synlogic, we’re programming microbes to treat complex diseases in the gut with the power of biology.
The Problem
Branched-chain amino acids such as leucine, isoleucine, and valine are essential amino acids that cannot be synthesized by the body. The human pathways for catabolizing branched-chain amino acids involve many highly regulated enzymes, and genetic defects in those enzymes result in a number of severe metabolic disorders. Synlogic is genetically engineering microbes to deliver critical functions that are missing due to such diseases. Prior to working with Ginkgo, Synlogic had been prototyping an enzyme pathway to consume leucine in E. coli Nissle. However, initial modeling suggested that more activity was needed for therapeutic effect.
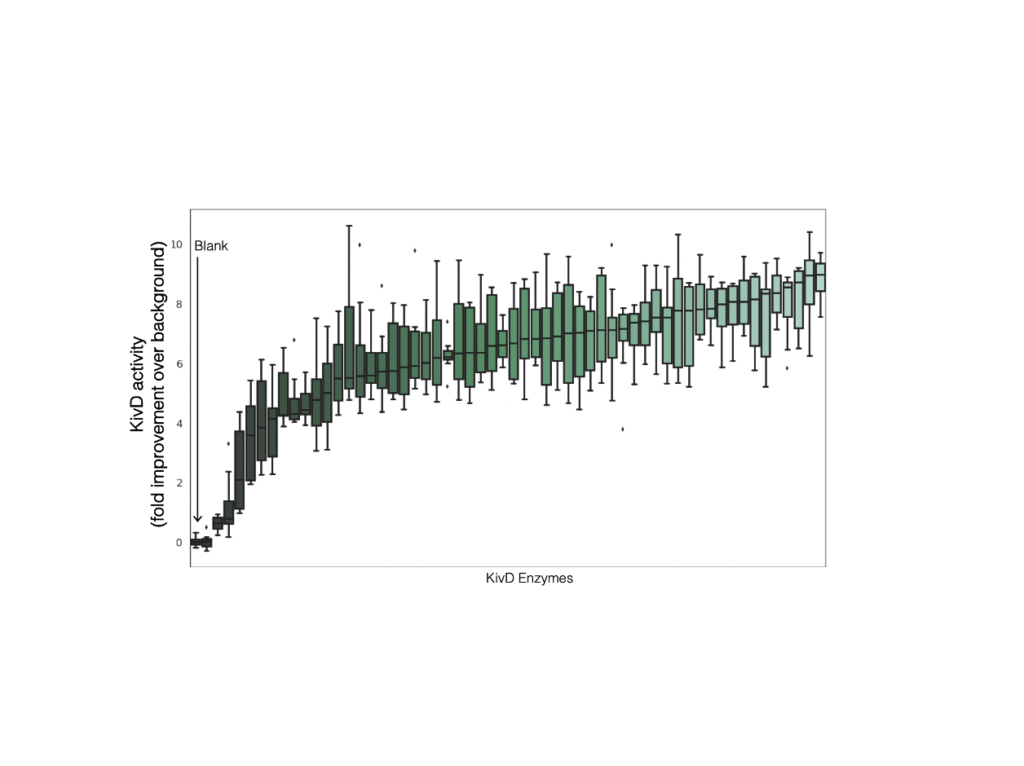
Enzyme Discovery
The pathway was initially designed with enzymes LeuDH from Bacillus cereus, KivD from Lactococcus lactis, and Adh from Saccharomyces cerevisiae. To optimize the complex pathway to increase consumption, Ginkgo sourced, synthesized and screened more than 1,200 homologs for each enzyme, designing each library to sample well beyond the enzyme sequence space that was described for these enzymes. The best performing enzymes had significantly more in vitro activity than the original enzymes used in the pathway. For example, the screening identified 5 Adh enzymes with >20x increase in activity relative to background activity. The top hits from each individual enzyme screening were assembled into a library of 350 operons, which were then screened with a high throughput leucine consumption assay and targeted metabolomics.
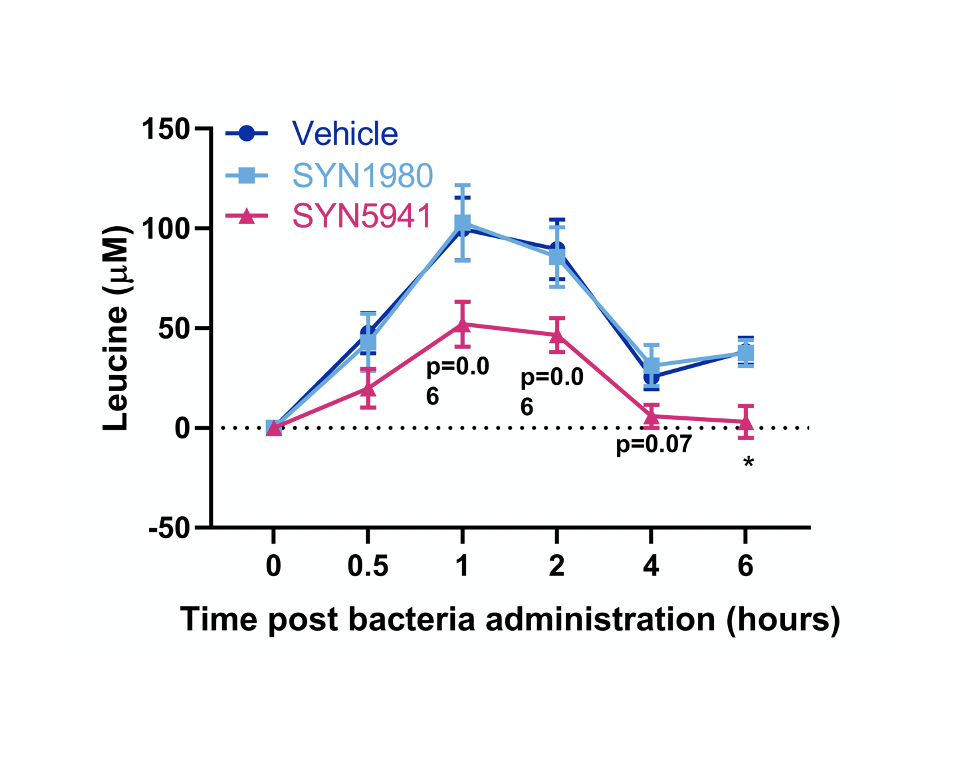
Compelling Pre-clinical Data
In the process of designing a better pathway, Synlogic discovered that Ginkgo’s optimized pathway performed better in an unmodified chassis strain. With a simpler biological design, Ginkgo improved the Synlogic strain’s ability to consume leucine by nearly 7x. After further screening and optimization by Synlogic, the Synlogic-Ginkgo optimized strain SYN5941 was also shown to significantly lower protein-induced leucine consumption in non-human primates.

Looking Forward
This work demonstrates the power of exploring a large combinatorial design space, and serves as an example of successful translation of in vitro improvement into in vivo biological activity for engineered probiotics. In 2019, Synlogic and Ginkgo further expanded their partnership with an equity investment and a range of new projects, expanding Synlogic’s pipeline and helping to accelerate more promising candidates to the clinic.
Recombinant DNA technology first made it possible to manufacture protein-based drugs nearly forty years ago, and today a large percentage of promising new drugs are biologics. We can only imagine what might be possible in the age of living programmable medicines.
For more information, visit synlogictx.com.
In Summary
- 1,200+
- Homologs screened for each enzyme
- 350
- Operons in library assembled from top hits from each individual enzyme screening
- 7x
- Improvement to the Synlogic strain’s ability to consume leucine
