We’re thrilled to have been awarded a 5-year contract alongside SaponiQx, Inc., totaling up to $31 million including program options, to discover and develop next-generation vaccine adjuvants.
This award comes to us from the Defense Threat Reduction Agency’s (DTRA) Joint Science and Technology Office (JSTO) for the Chemical and Biological Defense (CBD) Program, through the Medical CBRN [Chemical, Biological, Radiological, and Nuclear] Defense Consortium (MCDC) requirement 22-05, “Adjuvant Activity to Vaccines Prototype.”
We’ve been partners with SaponiQx in adjuvant discovery and development since 2021, and we’re honored to work with them on this project. Together, we will use a combination of high-throughput empirical and artificial intelligence/machine learning approaches, including Generative Molecular Design (GMD), to develop superior novel saponin-based adjuvants.
Adjuvants are components of vaccines that help to enhance the magnitude, breadth, and duration of the immune response to vaccination. Currently, only a handful of adjuvants are available for human use in licensed vaccines. SaponiQx’s STIMULON™ QS-21 is a key adjuvant component in market-leading vaccines for shingles, malaria, and respiratory syncytial virus. Novel adjuvants with enhanced properties, including tailored humoral and cellular immune responses, could pave the way for a new wave of innovative vaccines against existing and emerging pathogens.
The COVID-19 pandemic revealed the critical need for safe, effective, and accessible vaccines against emerging biothreat agents.
Imagine a future where vaccines are not only more affordable but also provide consistent protection in fewer doses, without causing discomfort or requiring refrigeration. We’re very excited by this opportunity to strengthen and expand the SaponiQx–Ginkgo partnership and to work with DTRA to make that future a reality.
Building on our achievements with STIMULON QS-21, SaponiQx is excited to realize our company’s founding vision of harnessing the potential of Generative Molecular Design to dramatically increase access to lifesaving vaccines around the world.
Rebecca Kurnat, Head of Operations at SaponiQx
We aim to demonstrate in the laboratory and in animal studies the ability of these novel adjuvants to protect against challenges from biothreat agents, such as the plague, and to provide lower cost, sustainable and scalable manufacturing processes by leveraging Ginkgo’s leading platform for cell programming. Together, we intend to design candidate adjuvants using SaponiQx’s leading platform for adjuvant generation, and to identify additional candidates by screening natural extracts for previously uncharacterized saponins and creating non-natural saponins with enzyme-based techniques. Harnessing a first-of-its-kind “data lake” for adjuvants, we plan to use iterative GMD to propose and optimize adjuvant structures against eight functional parameters. Adjuvant candidates will be put through in-depth testing, first in the laboratory for immune and toxicity responses, and then in studies of their effectiveness in protecting vaccinated animals from pathogens; QS-21 and the related QS-7 will serve as benchmarks.
By leveraging our leading platform for cell programming, we also intend to develop more affordable, sustainable, and scalable adjuvant manufacturing processes.
Ginkgo will develop a first-generation Adjuvant Development Candidate (ADC) production method, using a heterologous production strain such as brewers’ yeast, Saccharomyces cerevisiae. Our platform powers iterative Design–Build–Test–Learn-driven cell engineering to enable the rapid prototyping, optimization, and development of proteins, enzymes, metabolic pathways, and whole organisms under commercial-scale manufacturing conditions. Development of a first-generation ADC production method could facilitate further development of a sustainable mass-production manufacturing process for these complex adjuvants.
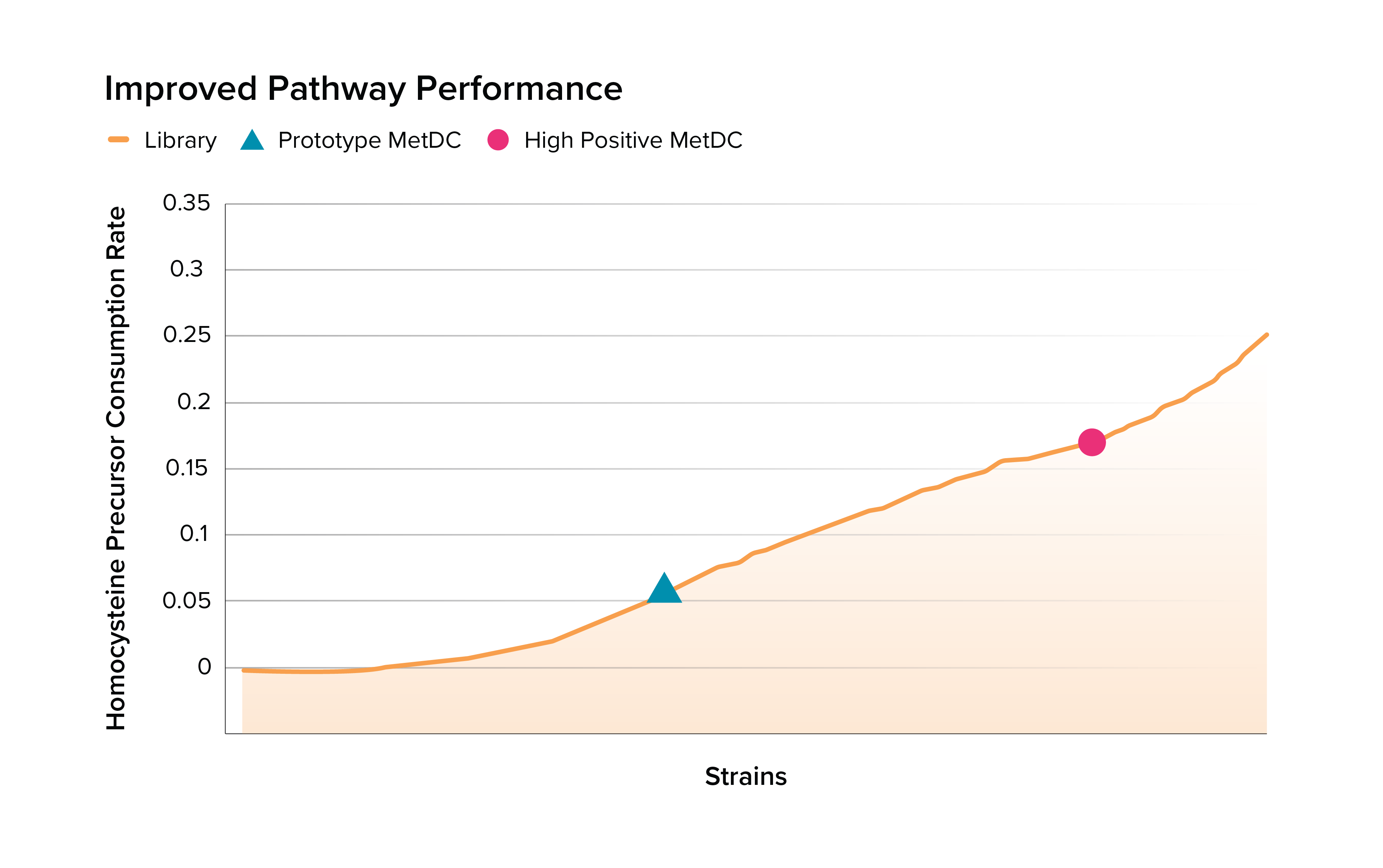
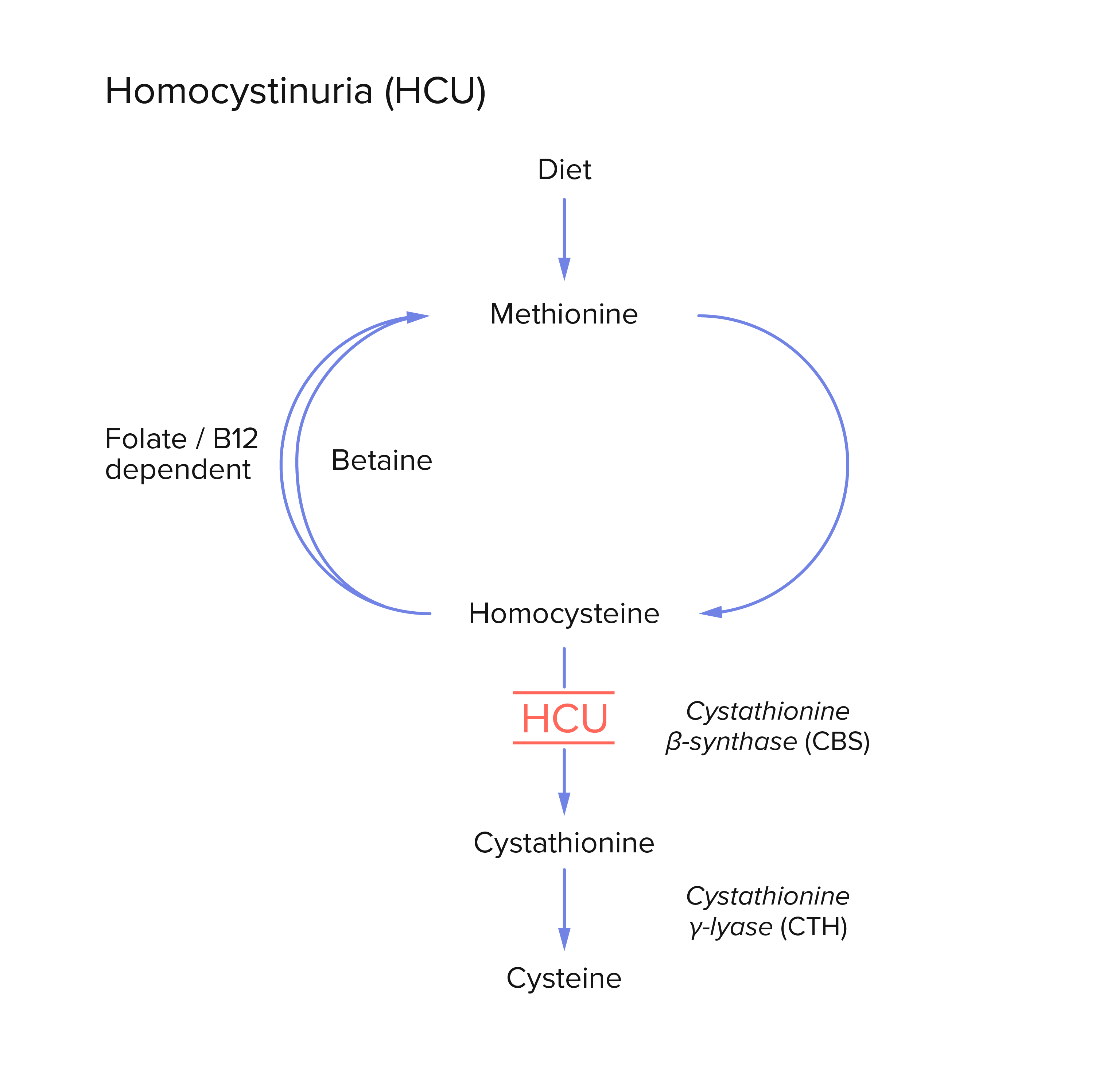 Solution | Boosting microbial metabolism for human disease
Solution | Boosting microbial metabolism for human disease