Watch the Event
On May 31st, Ginkgo hosted a live webinar to present our capabilities and latest results in CAR-T engineering and cell therapy.
A recording of the Virtual Event, Cell Therapy Services, is available here.
A Platform of Platforms for Full-Stack Engineering
Shawdee Eshghi, Senior Director of Mammalian Engineering, shared an overview of Ginkgo’s platform for mammalian cell engineering for biopharmaceutical applications. Advances in synthetic biology are revolutionizing medicine across a range of modalities and disease areas. By making biology easier to engineer, Ginkgo can help our customers improve efficacy, safety and access to these remarkable medicines.
For cell therapeutics, Ginkgo offers services in three categories, all supported by our industry-leading platform for DNA assembly, agile automation, and high-throughput screening in clinically relevant assays.
- Novel CAR Designs. Ginkgo has constructed libraries of novel ICDs, ECDs and armoring domains to optimize your CAR’s performance. We can perform pooled CAR screening in primary immune cells, offering both off-the-shelf assays and bespoke application-specific assays.
- Regulatory Elements. Tissue-specific promoters can improve the efficacy or the safety profile of your cell therapy. Strong and stable expression elements can increase potency. Ginkgo can assemble and screen large libraries of custom regulatory sequences.
- iPSC Engineering. Ginkgo’s platform can support the development of allogeneic cell therapies as well as more predictive in vitro models. We have extensive experience working with iPSCs coupled by our enabling platform technologies that include promoter screening, safe harbor discovery, directed differentiation optimization, and improved immune evasion strategies.
Combinatorial CAR Engineering at Scale
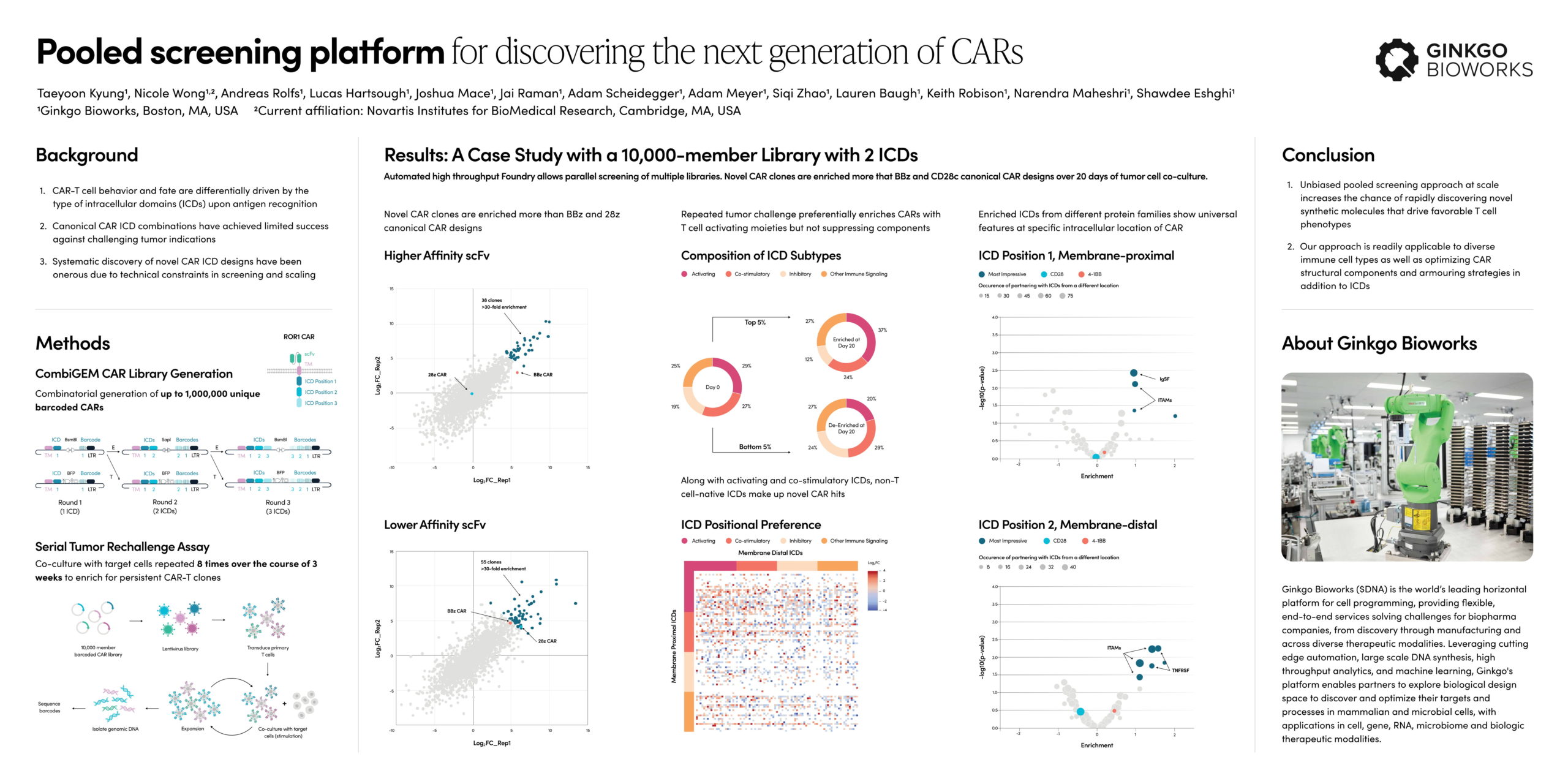
Taeyoon Kyung, Senior Mammalian Engineer, presented recent results from a foundry project to characterize 10,000 CAR intracellular domains (ICDs). Ginkgo identified multiple designs that outperformed standard CD28-CD3z and 41BB-CD3z designs in a serial tumor rechallenge model for T cell persistence. A range of follow-up assays were presented to characterize CAR4, a high performance design with improved proliferation, killing, and long-term fitness in challenging contexts.
This combinatorial CAR library screen demonstrates the potential of Ginkgo’s automation and design capabilities to deliver best-in-class cell therapeutics across a range of applications. The libraries developed for this project have been screened against suspension, adherent and spheroid target cells. A similar approach can be brought to bear to optimize CAR extracellular and intracellular domains in parallel in any given immune cell type. High throughput DNA synthesis and library assembly can support your R&D efforts across a range of therapeutically relevant synthetic proteins.
What Can Ginkgo’s Cell Therapy Services Do for You?
Ginkgo’s service offerings are well positioned to help R&D teams address a number of common challenges in creating an effective cell therapy: T-cell exhaustion, immunosuppression in the tumor microenvironment, antigen escape, cytokine toxicity.
For customers interested in our cell therapy offerings, we offer a range of partnership structures tailored to the scope and needs of your project. A direct evaluation and licensing model is available to put Ginkgo-built tech in your hands today. Alternatively, we can enter into an R&D collaboration to design, build, screen and optimize a cell-based therapeutic specific to your application. Your team will then have the option to evaluate and license the final designs. We look forward to working with you!