Overview
Cell therapy with CAR-T and beyond, transformed by synthetic biology
Advances in our ability to read, write, and edit DNA are transforming medicine. Cell therapies, which use engineered living cells to deliver therapeutic activity, are well positioned to benefit from these advances.
Ginkgo’s foundry brings together the full stack of mammalian cell programming capabilities needed to explore biological design space at scale. Our interdisciplinary team excels at designing and building complex libraries of all kinds. Our expansive automation infrastructure enables us to test these libraries in the most relevant cells and assays.
We offer capabilities to support discovery efforts for all cell-based therapies: autologous, allogeneic, iPSC-derived and beyond. For applications in allogeneic or iPSC-derived cells, we can enable partners seeking novel hypoimmune strategies and gene editing tools. Our complementary offerings in gene therapy can address challenges for CAR-T generation in vivo with viral or nonviral delivery.
Cell types
- Autologous
- Allogenic
- iPSCs
- in vivo
Solutions for
- CAR intracellular domain (ICD) optimization
- CAR binder domain discovery
- Armoring strategies
- Hypoimmune strategies
- Safe harbor site identification
- Differentiation process optimization
- Gene editor discovery & optimization
- gRNA and multiplexing optimization
- Cell-type specific promoter discovery
- Viral and non-viral vectors
01 CAR ENGINEERING
Combinatorial strategies for next- generation CAR designs
Early breakthroughs in CAR (chimeric antigen receptor) design demonstrated the power of genetic engineering to create novel cellular functions. More than 20 years later, many emerging cell therapies still rely on the same few functional domains developed in the early days.
Ginkgo brings novelty and scale to our partners’ CAR discovery programs. Our platform enables us to sample CAR domains with a variety of functional roles, structural positions, and sourced from diverse immune cell types. The result is an expansive and unbiased approach to discovery that deeply samples the breadth of therapeutic activities a CAR can produce.
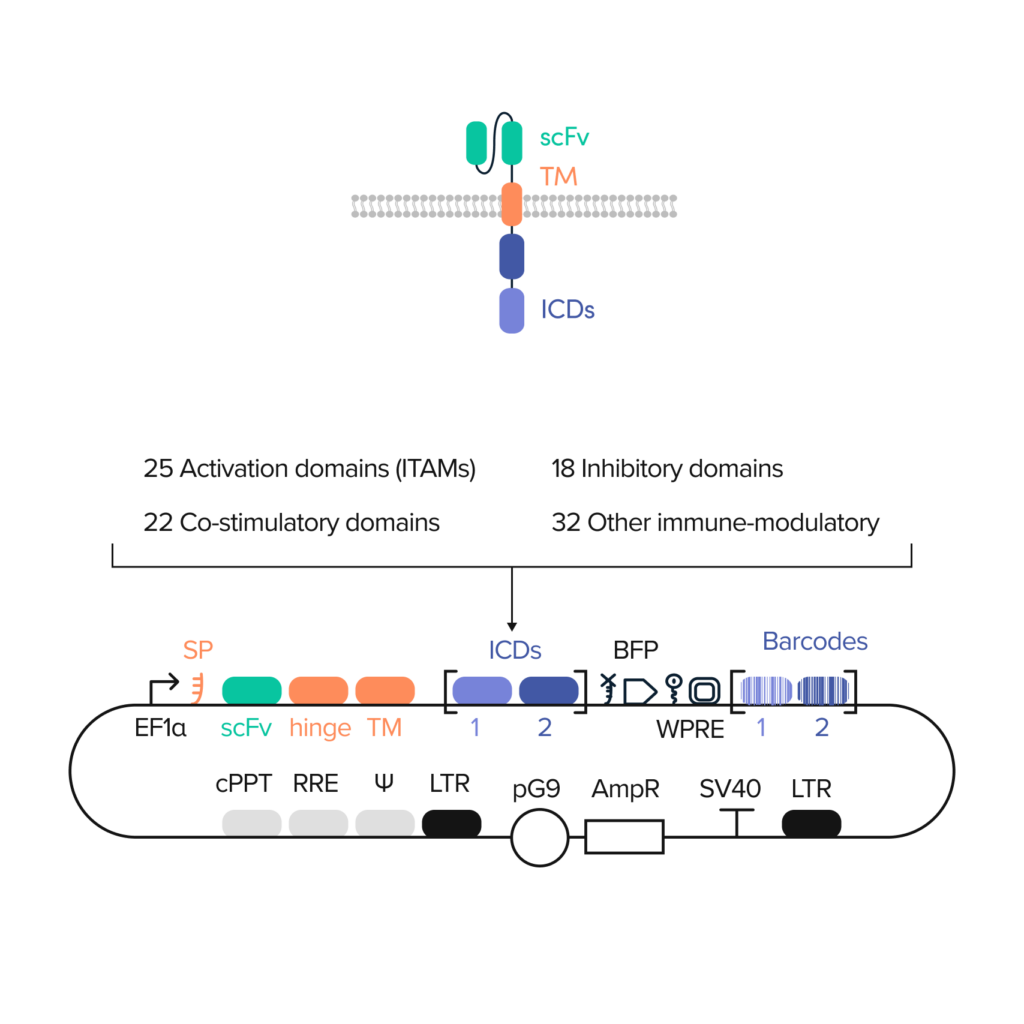
An example CAR diversity library from the Ginkgo foundry
With the power to design and build DNA at scale, we can combinatorially explore large numbers of functional CAR domains.
Ginkgo can create CAR libraries with two or three combinatorial ICDs and as many as 104 to 106 unique designs.
DNA barcoding tracks the performance of individual constructs in cell-based assays at high resolution.
Representative numbers from a recent CAR design campaign
(Screen designs are project-specific and results may vary)
- 4x, 10,000 member CAR design libraries
- 2x PBMC donors
- 3x tumor cell lines
- 3x replicates
- = 720,000 replicates in a single pooled screen
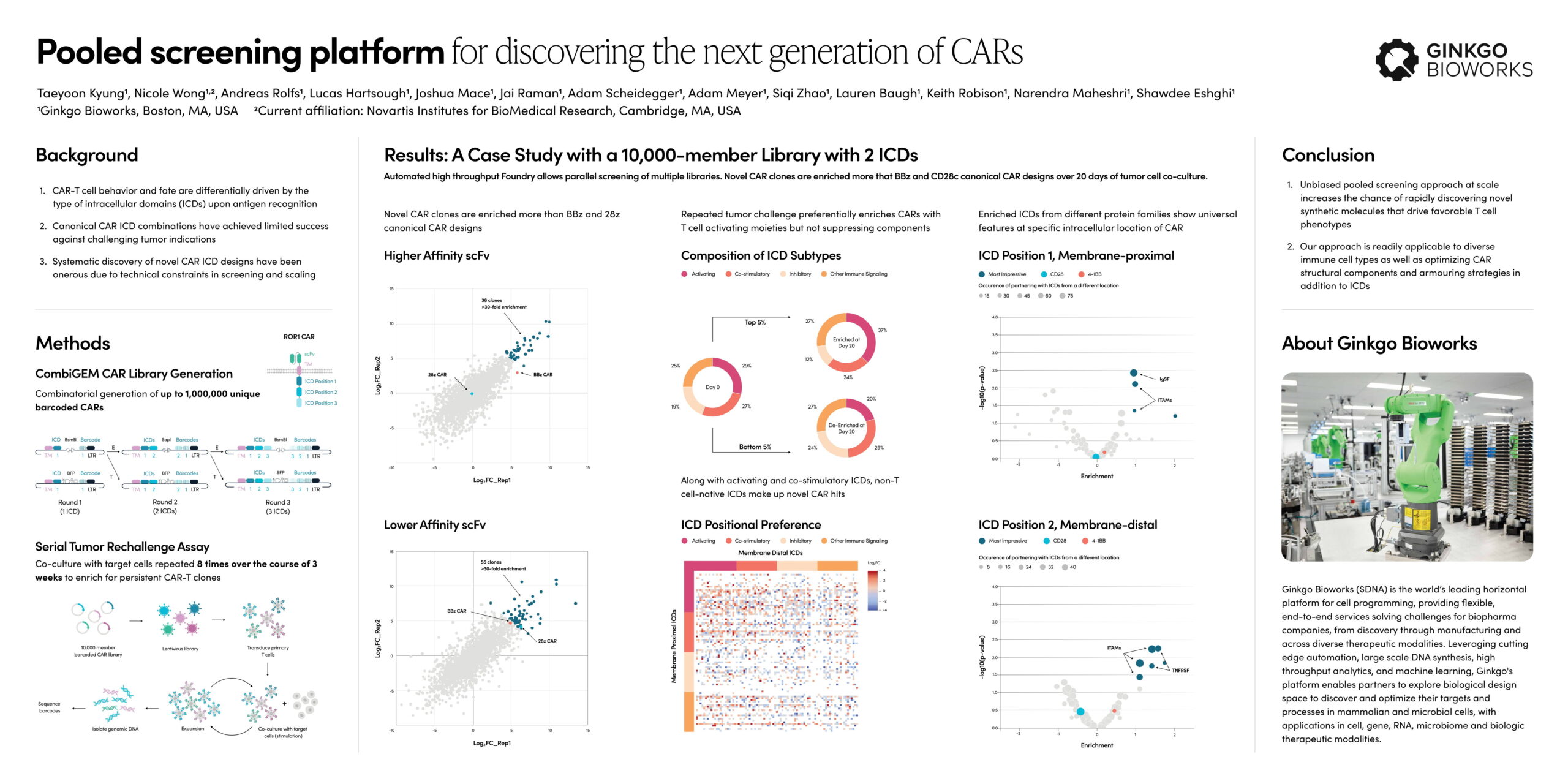
Case Study
Combinatorial and pooled screening of a 10,000-member CAR library identified dozens of ICDs that outperformed canonical designs including BBz and 28z
Pooled screening platform
02 IPSC ENGINEERING
Comprehensive cell programming capabilities for novel strategies and cell lines
Beyond the CAR-T paradigm, Ginkgo brings to our partnerships a versatile set of tools for cell engineering and the human experts to deploy them effectively. Our capabilities support work with allogeneic, donor-derived cells, and induced pluripotent stem cells (iPSCs).
The mammalian cell engineering platform at Ginkgo was developed to address our partners’ most pressing needs: persistence, efficacy, safety. For allogeneic cell therapies, we can explore hypoimmune strategies addressing both the innate and adaptive immune responses.
Engineered cell therapies require predictable performance from stably integrated therapeutic cassettes. We can perform extensive safe-harbor and promoter screening to identify the genetic constructs for therapeutic activity that are strong, sustained and cell-type specific.
All cell-based therapeutics benefit from process optimization to address manufacturing challenges. Our capabilities in automation and design of experiments (DOE) allows us to efficiently optimize complex cell differentiation processes.
Solutions for
- Hypoimmune strategies
- Genomic safe harbor site identification
- Promoter screening
- Differentiation process optimization
03 GENE EDITING
Gene editor discovery and optimization
Novel cell therapies are often driven by the development of novel gene editing tools. The spectacular rise of CRISPR and other gene editing systems have enabled a wide range of modifications to DNA and RNA sequences, unlocking new therapeutic possibilities.
Ginkgo’s partners gain access to an extensive set of assets and capabilities to enable the development and optimization of novel gene editors. Work with our team of experts to co-create a discovery project for your application.
Metagenomic Discovery
~2 billion searchable gene sequences in our proprietary databases
In-house DNA synthesis specializing in difficult sequences
Extensive experience in combinatorial and barcoded library assembly
Protein Engineering
Owl, a proprietary software platform for AI-guided protein engineering
Experience in running more than 400 engineering campaigns for diverse applications
Extensive data generating capacity for training AI models
Cell
Engineering
Experience with 60+ cell types, including primary cells and iPSCs
Automated workcells for transfection and nucleofection enabling 1000s of parallel assays
Functional and sequence-based readouts with high-throughput screening or next-gen sequencing
HOW WE PARTNER
An innovation partner with tech you can’t get anywhere else
Ginkgo Bioworks is a pure platform company. We’re dedicated to helping our partners to advance their cell therapies rather than seeking to develop our own products in house. We offer a variety of partnership models to meet your technical needs, whatever your stage of the R&D process.
Our partners gain access to our industry-leading foundry automation infrastructure, our large libraries of partially and extensively characterized genetic assets and our world-class team of experts in mammalian cell engineering.
Why work with Ginkgo to develop your next cell therapy?
- Take control of your R&D spending by replacing fixed costs with variable costs.
- Avoid costs and delays associated with building your own infrastructure.
- Launch faster by starting from pre-characterized genetic elements.
- Gain access to our unique biological assets and industry-leading capabilities.
- Work with our team of dedicated experts across all stages of discovery.
Ginkgo is ready to partner with you to enable the next generation of cell therapies!
"*" indicates required fields