Overview
AAV capsid assets with proven performance, engineered at foundry scale
Ginkgo brings together everything you need to build the next generation of gene therapies: assets to build on and capabilities to build with. Launch your project quickly using one of our pre-characterized AAV capsids or put our foundry to work on your new or improved capsid designs.
Our licensable assets include a range of off-the-shelf capsids with extensive characterization data in mouse models and non-human primates (NHPs). Our capabilities include deep expertise in DNA and protein engineering, industry-leading infrastructure for automation and high-throughput screening, and technologies for pooled and barcoded assays in animal models.
We offer end-to-end capabilities that can support your R&D project from discovery to production. Build manufacturability into your gene therapy designs from day 1, then scale up your production with our proprietary plasmids and host strains.
Capsid Engineering

Payload Engineering
AAV Production
Solutions for
- Cell-type specificity
- Tissue tropism
- Enhanced potency
- Neutralizing antibody escape
- Liver de-targeting
- Capsid manufacturability
- AAV production hosts
Impacts for patients & R&D partners
- Reduce side effects
- Lower dose requirements
- Minimize immunogenicity
- Cut production costs
- Reduce batch variability
- Improve purity & quality
- Help derisk translation from animal models to humans
01 CAPSID ASSETS
The STRV capsids: relevant functionality, validated in vivo
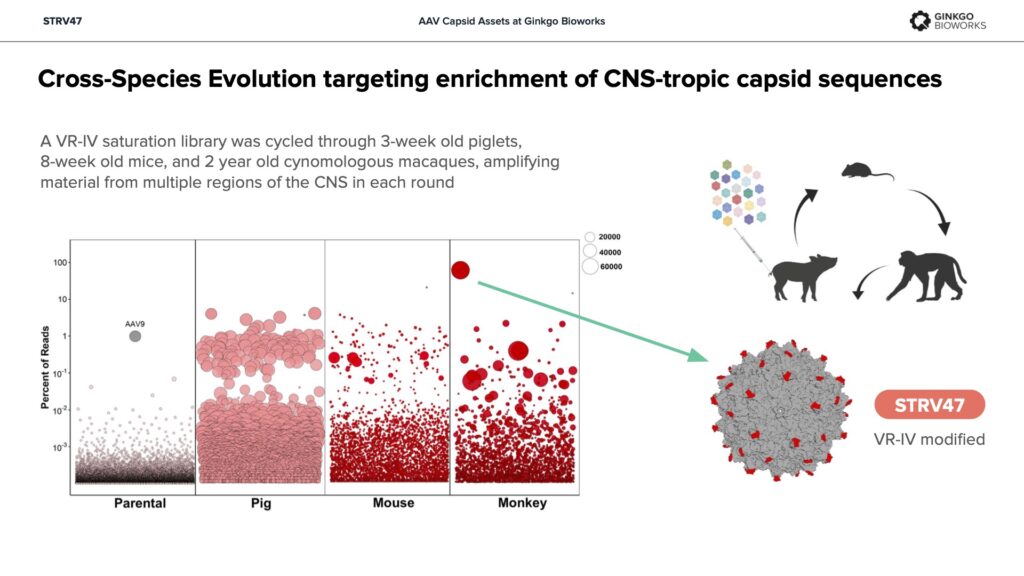
Ginkgo acquired the STRV capsid library from StrideBio in 2023 as both a collection of proven assets and a resource for generating new capsid designs. The STRV capsids were created to overcome key limitations in the first generation of gene therapies including the seroprevalence of neutralizing antibodies, excessive liver tropism and imperfect translation from preclinical model systems to human patients.
Derived from a variety of AAV serotypes, the STRV capsids have undergone extensive structural engineering to reduce their antigenic footprint and detarget the liver while preserving key stabilizing motifs that support manufacturability. To help derisk clinical translation, the STRV capsids were evolved through multiple species during development for improved cross-species compatibility.
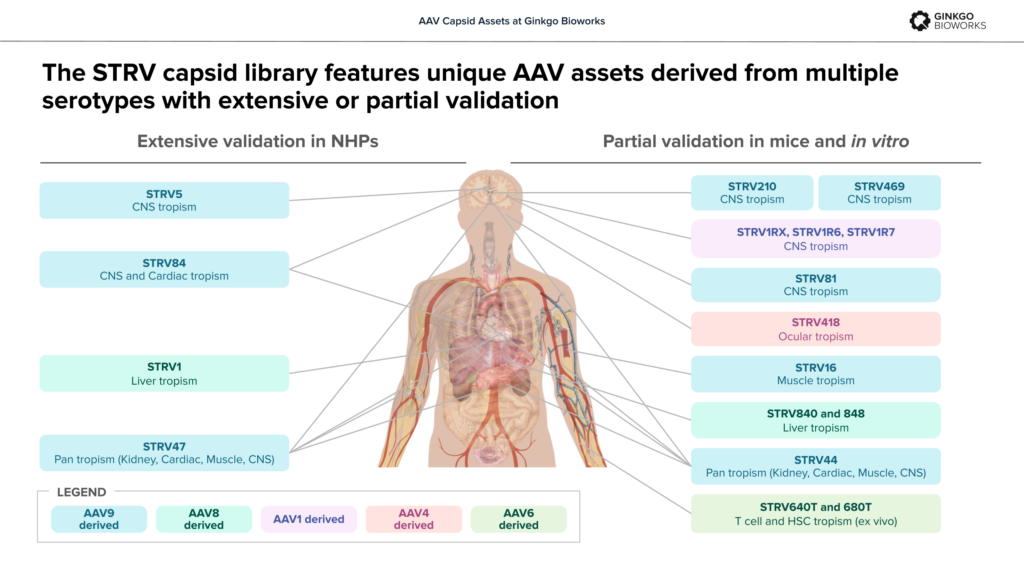

Learn more about individual STRV capsids
STRV47 – Pan-tropic, validated in NHP models for high potency in kidney.
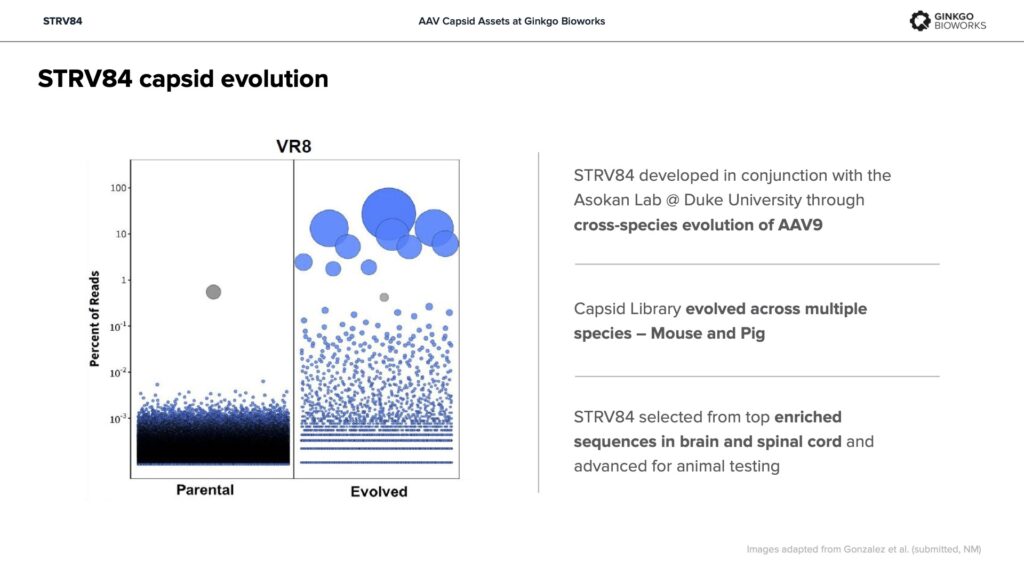
STRV84 – Enhanced cardiac and CNS tropism, liver de-targeting in multiple animal models.
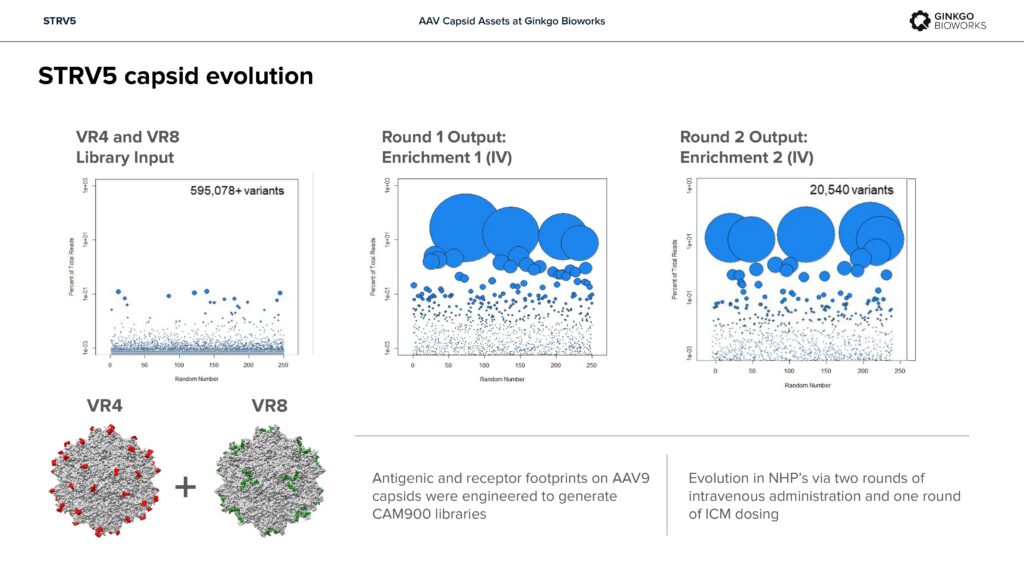
STRV5 – Potent CNS transduction in NHPs.
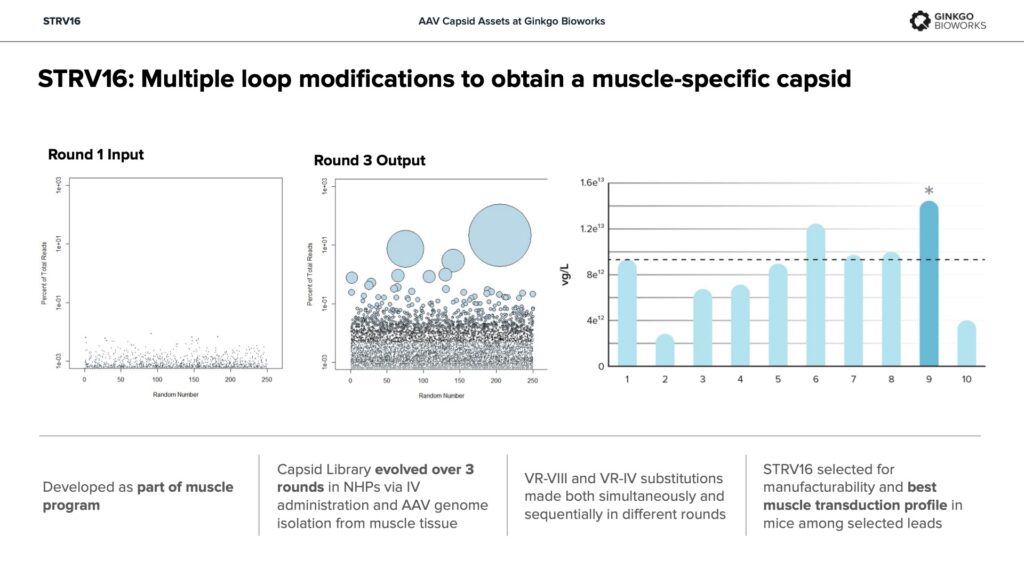
STRV16 – Selectivity for skeletal muscle vs. heart, liver de-targeting

02 CAPSID ENGINEERING
Build new epitopes for desired functionalities, informed by massive datasets
New capsid engineering projects shouldn’t have to start from scratch. Leap-frog the early stages by drawing from our extensive dataset of partially and extensively characterized AAV capsid designs.
Data-guided design can mean the difference between exploring huge speculative design spaces in the dark and building focused libraries that are pre-filtered for your application.
Key engineering targets include tissue tropism, cell-type specificity, potency, and route of administration.

Foundry capabilities for AAV engineering R&D projects
- Design capsid libraries informed by previously characterized AAV variants.
- Build 1000s of barcoded capsids in parallel with Ginkgo’s state-of-the-art foundry automation.
- Test constructs with a variety of in vitro assays, or perform pooled biodistribution studies in NHPs.
- Characterize performance at single-cell resolution with DNA, RNA and protein read-outs.
03 PAYLOAD ENGINEERING
Discover regulatory elements for improved tissue specificity and safety
Cell-type specificity is key to improving safety and efficacy profiles in gene therapy, minimizing dose requirements while maximizing therapeutic activity. Ginkgo has the capacity to screen cell-type specific regulatory elements in vivo, at scale. We can design and build libraries of up to 1 million targeted regulatory elements for parallel testing in a single pooled screen.
Integrated design strategies, in which AAV capsids and genetic payloads are engineered in parallel, can be more effective than optimizing individual genetic elements in isolation. Ginkgo’s extensive capabilities in DNA synthesis and high-throughput automation can unlock a more holistic approach for our partners, helping to identify globally optimized gene therapies.
CUPID (CUstom Promoter IDentification) is our proprietary technology for regulatory element discovery. By using circularizing RNA constructs as expression reporters, we can reduce experimental noise caused by RNA degradation. The result is a clearer picture of regulatory element performance.
Large regulatory element libraries
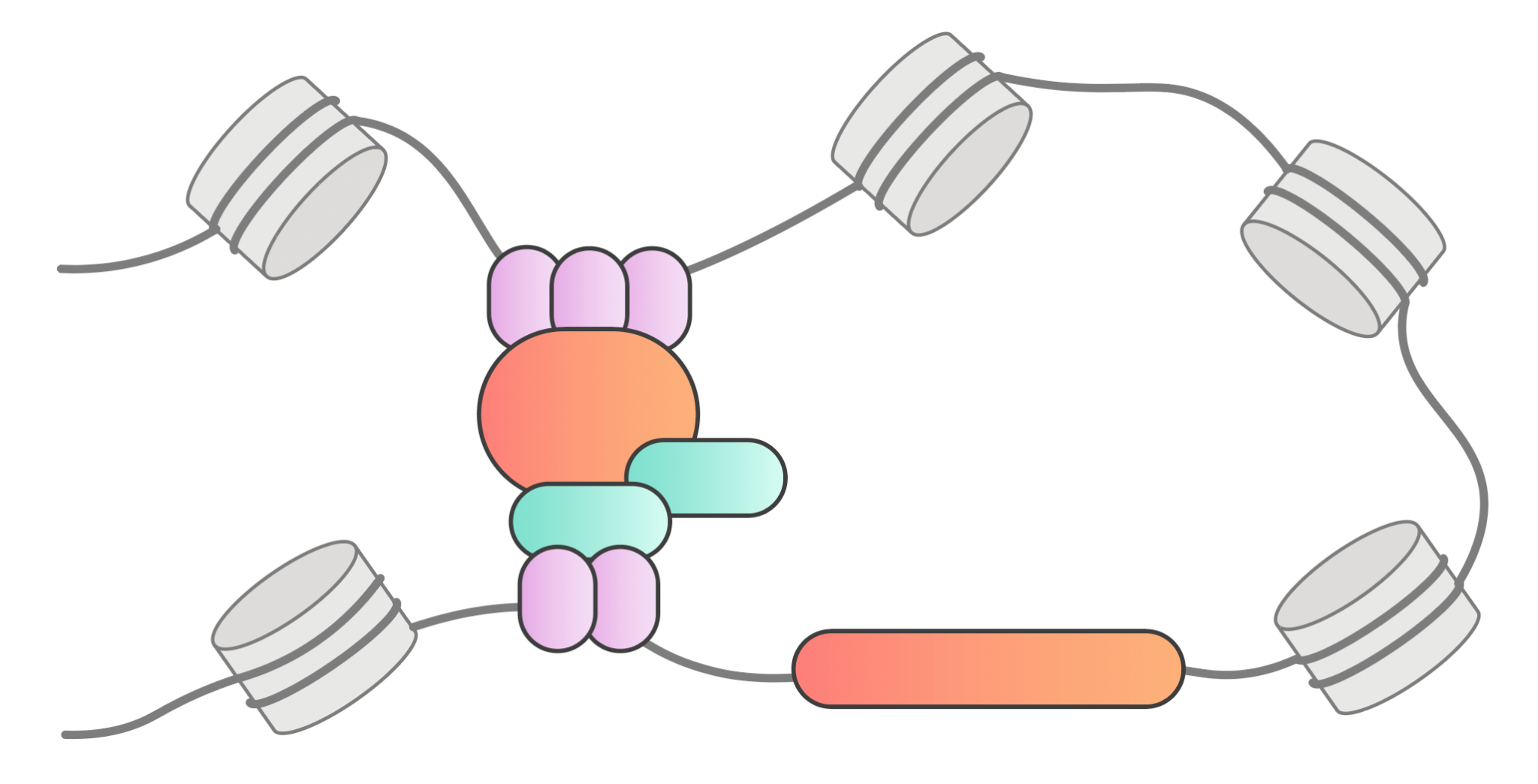
We extensively sample candidate regulatory elements including promoters, enhancers, repressors, or combinations thereof. Each regulatory sequence drives expression of a barcoded, self-circularizing CUPID element.
Pooled screening in cell or tissue models
We deliver the barcoded library with either with plasmids or AAV, allowing us to charcterize targeting or de-targeting in cell lines, primary cells, organoids, and in vivo models.
Precise readouts with circRNA reporters
We quantify the resulting barcoded circRNA reporters with next-gen sequencing to identify top performing promoters within our partners’ specific context.
04 PRODUCTION
Improve AAV titers with solutions for transient transfection workflows or stable cell lines
Ginkgo offers a range of assets and capabilities to help our partners accelerate and derisk the AAV production process. By leveraging the power of the Ginkgo foundry, we can explore large combinatorial design spaces to identify plasmids and cell lines with improved productivity. We can optimize transformation protocols, production conditions, expression constructs, integration sites, host genetic backgrounds and more.
Optimize host cell lines for transient transfection
Leverage our HEKMO clonal host cell line, or improve your own host
Identify knockout, knockdown, or overexpression targets with high-throughput transcriptomics data from 1000s of gene candidates
Evaluate genomic and functional titers and select top-performing clones via single-cell cloning
Optimize plasmids for transient transfection
Explore plasmid miniaturization by systematically eliminating unnecessary sequences
Screen 100s of Rep-Cap plasmid designs to identify a combination that improves titers
Co-develop double- or single-plasmid systems to simplify transfection
Implement a DoE approach to optimize AAV production across plasmid ratios, transfection reagents, additives, etc.
Develop stable cell lines
Improve a host cell line by screening knockout, knockdown, or over-expresson of 100s of targets
Identify “safe harbor sites” for genomic integration
Develop an inducible control system to minimize the toxicity of viral genes
Improve cell vigor by identifying smart design spaces that minimize toxicity
Impacts for production optimization
- Improve consistency and predictability
- Optimize production titers
- Improve full-empty ratios
- Reduce COGS
- Help ensure scalability
HOW WE PARTNER
An innovation partner with tech you can’t get anywhere else
Ginkgo Bioworks is a pure platform company. We’re dedicated to helping our partners to advance their AAV-based gene therapies rather than seeking to develop our own biopharma products in house.
Our partners gain access to our proven AAV assets, our massive datasets featuring partially or extensively characterized capsid designs, our proprietary circRNA reporter technology, and our industry-leading foundry automation infrastructure.
Why work with Ginkgo to develop your next gene therapy?
- Take control of your R&D spending by replacing fixed costs with variable costs
- Avoid costs and delays required to build your own infrastructure
- Launch faster by starting from pre-characterized genetic elements
- Gain access to our unique gene therapy production assets
- Work with our world-class team of synthetic biology experts
We offer a variety of partnership models, whatever your stage of the R&D process.
- License our existing capsids for your indication and target
- Evaluate capsids under a MTA prior to exercising an option to license
- Engage in a research collaboration to discover novel capsids or regulatory elements.
Ginkgo is ready to partner with you to enable the next generation of gene therapies!
"*" indicates required fields