Interested in leveraging Ginkgo Enzyme Services for your R&D? Get in touch here!
Executive Summary:
Synlogic designs and develops Synthetic Biotics — living biotherapeutics based on engineered bacteria — with the potential to provide safe, convenient, orally-administered, non-systemically absorbed new medicines for serious diseases. The company has a focus on rare metabolic diseases, including homocystinuria (HCU), which is characterized by elevated levels of total homocysteine (tHcy). Patients are at risk of both acute, life-threatening events and severe complications. HCU treatments aim to reduce tHcy levels in patients, firstly through limiting intake of methionine, an amino acid precursor to homocysteine found in many foods. Synlogic recognized the potential of methionine as a target for a Synthetic Biotic and partnered with Ginkgo to improve the activity of their initial prototype biotherapeutic candidate.
Synlogic and Ginkgo set out to increase the activity of methionine degradation, focusing on the key enzyme Methionine Decarboxylase (MetDC). Leveraging Ginkgo’s Enzyme Intelligence suite of tools, Ginkgo delivered genetic parts that multiplied methionine degradation activity in vitro, including a new MetDC enzyme with more than four-fold higher activity. Within a year of initiating the program, Synlogic incorporated these components into their prototype strain and evaluated the activity of the optimized strain, SYNB1353, in mice and nonhuman primates.
Opportunity | Developing living medicines for a genetic disease
Based in Cambridge, Massachusetts, and founded in 2014 by MIT professors Jim Collins and Tim Lu, with support from Atlas Ventures, Synlogic designs living medicines for diseases with significant unmet needs. The company uses synthetic biology to genetically engineer probiotic bacteria (Escherichia coli Nissle 1917) and generate living medicines designed to metabolize or synthesize validated biological targets of known disease pathophysiology.
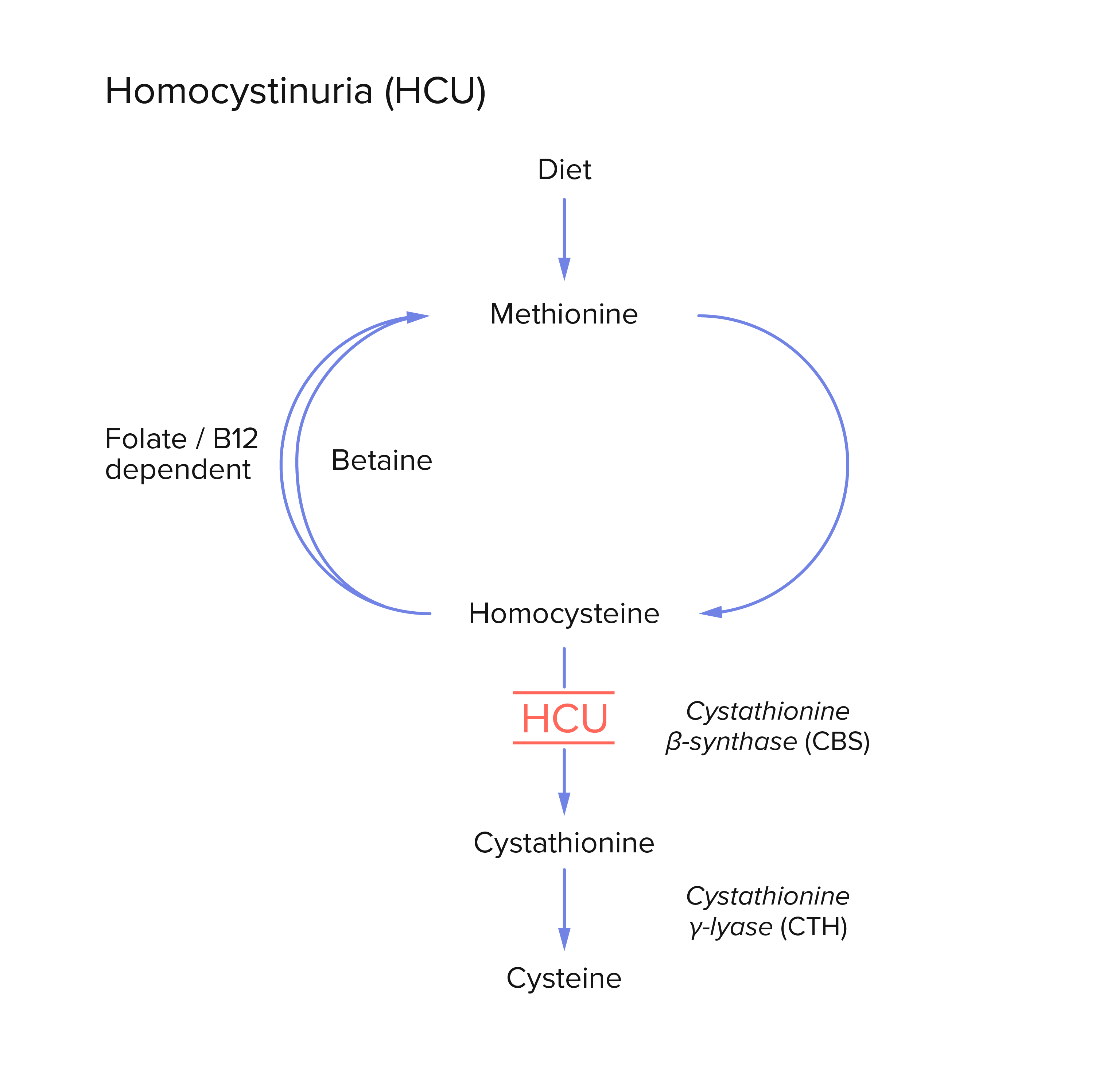
One disease Synlogic is targeting is homocystinuria (HCU), an inherited disorder characterized by risks of elevated homocysteine. The build-up of homocysteine can lead to multiple adverse effects, including bone defects, intellectual disabilities, and life-threatening blood vessel obstructions. Treating HCU aims to lower levels of total homocysteine (tHcy); a cornerstone of treatment is restricting dietary methionine, an amino acid found in protein-containing foods, and which is a precursor of homocysteine.
Joanna, who lives with HCU and has endured two strokes, seizures, and vision impairment because of this disease said, “We need to come together as a community and do more. Nobody knows how devastating HCU can be… We must continue to focus on research and work to develop new treatments. In the meantime, I want those living with HCU to know they are not alone and there are people who want to help.”
Current treatment options for HCU are limited in terms of safety, tolerability, and efficacy, underscoring the need for a new, innovative approach: Synlogic engineered a Synthetic Biotic to target and metabolize methionine as a means of lowering tHcy levels to treat HCU.
 Solution | Boosting microbial metabolism for human disease
Solution | Boosting microbial metabolism for human disease
In developing a Synthetic Biotic for HCU, Synlogic sought to improve two activities of their bacterial strain: the methionine importer that transports methionine into the bacterial cell, and the methionine decarboxylase (MetDC) that efficiently converts methionine into non-toxic compounds to prevent tHcy accumulation.
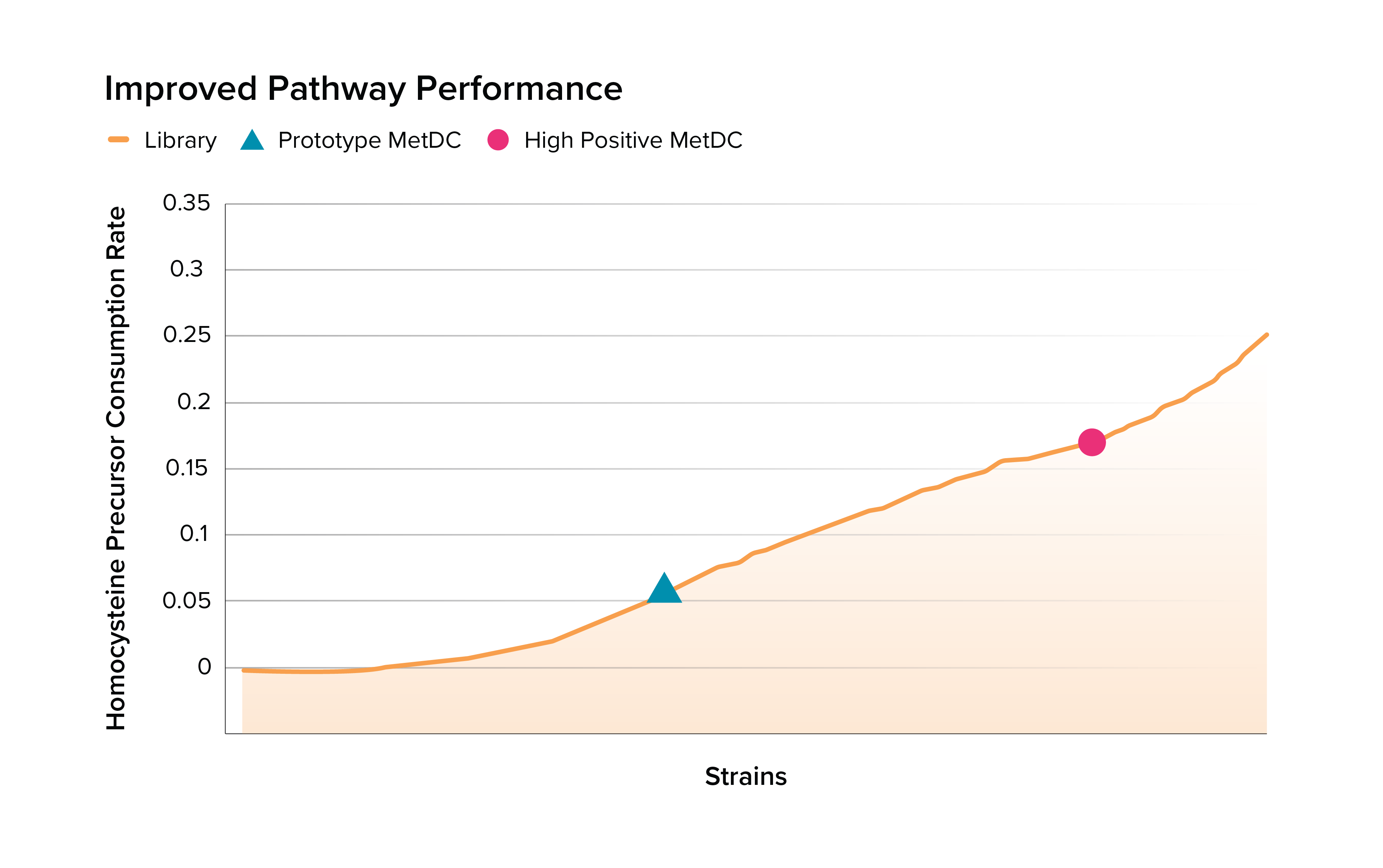
Ginkgo’s protein engineering team took two approaches to improving these components. A metagenomic approach searched through Ginkgo’s extensive multi-billion protein database for a biologically diverse set of candidate enzymes. In parallel, a protein engineering approach applied predictions from machine learning models in combination with structural analysis to design variants of the two proteins with a high likelihood of improved functionality. Combined, these approaches identified roughly 2,000 candidate methionine decarboxylases, and a targeted library of roughly 150 candidate methionine importers.
DNA for the enzyme libraries was synthesized and transformed into Synlogic’s strain background. In parallel, Ginkgo’s engineers developed a bespoke, high-throughput assay that would test the functionality of the importer protein and MetDC proteins. Once the DNA was synthesized and transformed into the screening background and the bespoke assay was on-boarded onto Ginkgo’s high-throughput automation platform the strains were screened in high-throughput in Ginkgo’s Foundry. The team identified an importer and decarboxylase that, in combination, showed significant improvement over Synlogic’s previous prototype. Synlogic was able to verify that these two new proteins improved their strain’s methionine degradation in vitro.
Outcome | Developing new treatments for human disease
Within a year, Ginkgo’s collaboration with Synlogic resulted in the naming of the investigational new drug, SYNB1353. The drug successfully demonstrated proof-of-mechanism in humans by showing the ability to degrade methionine and reduce its plasma levels using a dietary model. This is the first investigational new drug developed on Ginkgo’s platform. It was granted Orphan Drug Designation, Rare Pediatric Disease Designation, and Fast Track designation by the FDA. Next steps for the program include a Phase 2 study in patients with HCU. SYNB1353 offers a potentially new, orally administered, and non-systemic approach to degrade methionine, thereby lowering tHcy and its associated risks and daily disease burden of HCU.
“As a company, we are in regular contact with leaders and members of the HCU community, who continually express their appreciation for our efforts to bring something new forward,” said Mylene Perreault, PhD, Head of Research at Synlogic. “It is very powerful to hear directly from patients and caregivers about how transformative it would be to have an option that could target and convert methionine, lowering total homocysteine levels in the safe and convenient way that SYNB1353 could provide. Ginkgo has been an important part of this program’s journey and the collaboration that has helped us reach the point of studying this new drug candidate in patients.”
